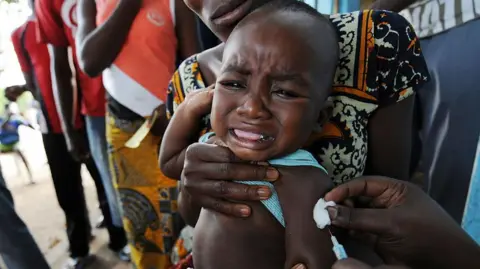
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticised by the World Health Organization as 'unethical'. The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age.
The WHO said it had 'significant concerns' about the plan, and described the birth-dose vaccine as 'an effective and essential public health intervention, with a proven record'.
The US health department, headed by Robert F. Kennedy Jr., who has questioned the effects of vaccines, had intended to use the trial to answer questions about the jab's broader health effects.
The WHO expressed concerns regarding the study's scientific justification, ethical safeguards, and consistency with established standards for research involving humans. The organization stressed that the jab had been used for more than three decades in over 115 countries.
It stated that giving a proven life-saving intervention to some newborns but not others exposed them to 'potentially irreversible harm'. The WHO noted that a significant portion of Guinea-Bissau's population has hepatitis B and that vaccination at birth prevents the virus from being transmitted from mother to baby in 70-95% of cases.
Public outrage over the project led to the Guinea-Bissau government suspending it last month. Critics question why newborns were chosen for the trial, especially as it comes amid shifts in US vaccination policies.
The WHO recommends that all newborns receive the hepatitis B vaccine within 24 hours of birth, pointing out that infection at birth is the most common route to lifelong infection. This controversy brings to focus ethical standards in vaccine trials, especially in vulnerable populations.