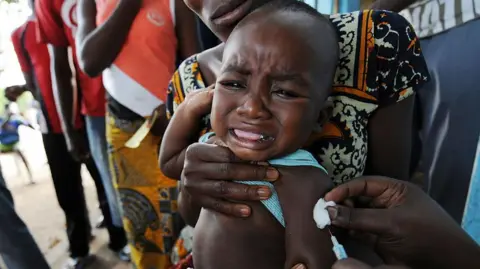
WHO Condemns Controversial US-Funded Vaccine Trial in Guinea-Bissau
The World Health Organization has criticized a proposed hepatitis B vaccine trial for newborns in Guinea-Bissau, calling it unethical and raising concerns about its scientific justification.
This US-funded study aimed to analyze the effects of administering the hepatitis B vaccine at birth compared to delaying it until the baby was six weeks old. WHO has raised alarms over the ethical implications of selecting which infants would receive the vaccine, citing the risks of exposing some to preventable diseases while leaving others unprotected.
The ethical concerns stem from the potential for irreversible harm to those who would not receive the vaccine, which has a proven track record of preventing hepatitis B transmission from mother to child in a majority of cases. Dr. Robert F. Kennedy Jr., who heads the US health department, had intended to use the study to investigate broader health effects of the vaccine.
Under pressure from public demand, the Guinea-Bissau government suspended the trial last month, which would have involved around 14,000 newborns. Critics, including the former health minister of Guinea-Bissau, have expressed disbelief that babies in a vulnerable population were targeted for this trial, emphasizing that Guinea-Bissauans should not be treated as test subjects.
With over 12% of adults in Guinea-Bissau estimated to carry chronic hepatitis B, the WHO has strongly endorsed immediate vaccination at birth, asserting that such preventative measures are essential to curb the spread of this life-threatening virus.