The newly formed vaccine advisory panel led by US Health Secretary Robert F. Kennedy Jr. is under intense scrutiny as it prepares to review established immunization schedules for children and teens. The Advisory Committee on Immunization Practices (ACIP) held its inaugural meeting on Wednesday, just weeks after Kennedy dismissed all 17 prior members of the committee, which recommends vaccination protocols to the Centers for Disease Control and Prevention (CDC).
The latest members of ACIP include individuals with ties to vaccine skepticism, prompting public health experts and political figures to voice concerns about their qualifications. During the meeting, the panel's chair, Dr. Martin Kulldorff, revealed he was previously terminated from his teaching position at Harvard for refusing to receive a COVID-19 vaccine. He announced plans for new working groups that will investigate childhood vaccination schedules, along with the reassessment of vaccines that gained approval over seven years ago, including the longstanding hepatitis B vaccine administered to newborns.
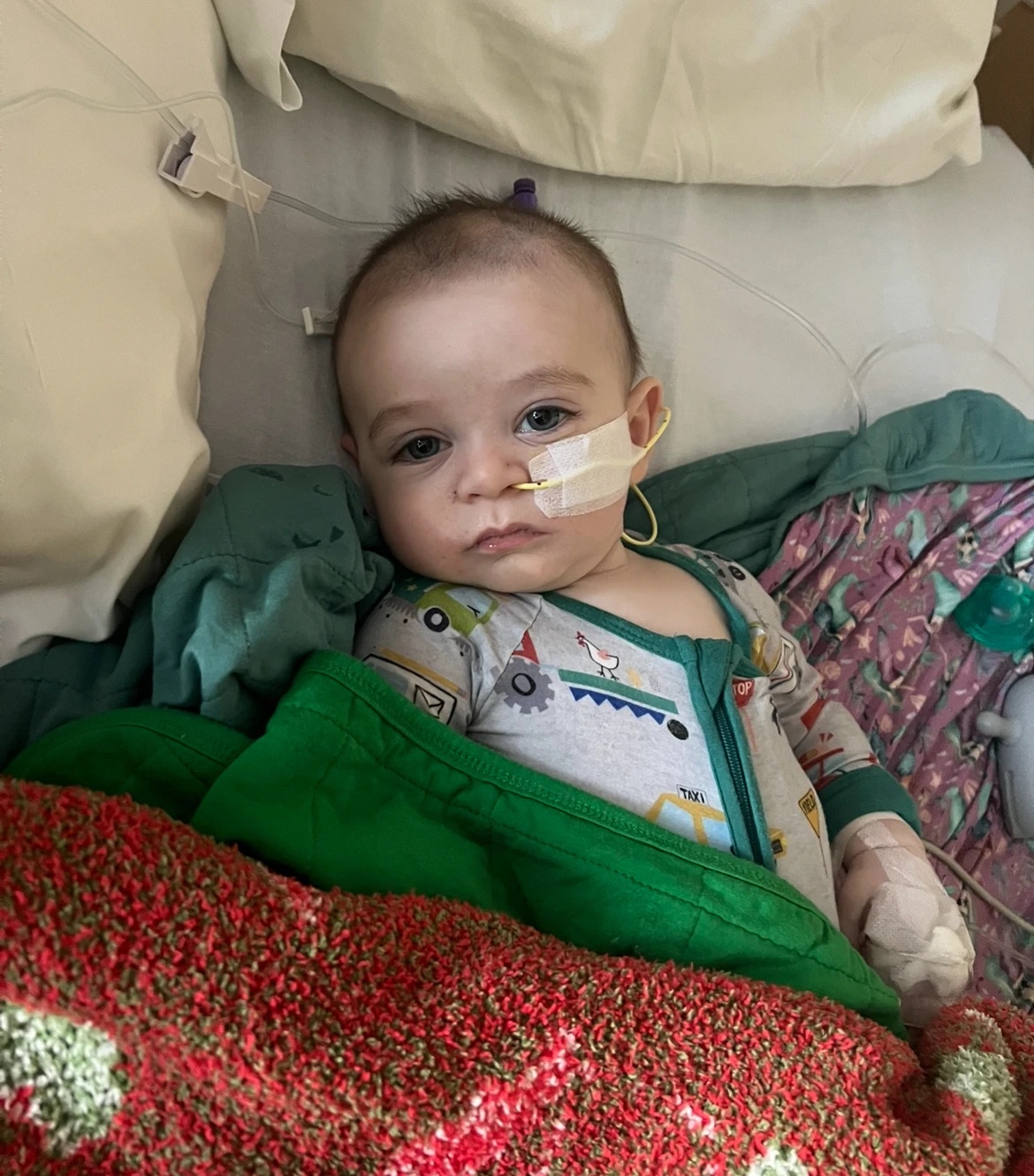
Concerns surrounding this review are substantial, particularly as critics surmise it undermines the credibility of approved vaccines. Bill Hanage, a professor at Harvard TH Chan School of Public Health, stated that questioning the approval processes of these longstanding vaccinations lacks rational justification. The panel was expected to cast votes regarding new recommendations for RSV, a respiratory virus posing risks to infants, but this agenda item has been postponed.
As part of the meeting, the panel also planned to discuss thimerosal, a mercury-containing preservative that has not been included in most vaccines for many years. This choice has puzzled experts, including Dr. Hanage, who expressed frustration that the new committee lacks the diverse expertise seen in prior panels. Former ACIP member Dr. Paul Offit criticized Kennedy's selection process, indicating that members predominantly share an anti-vaccine stance similar to Kennedy's.
Further criticism comes from Republican Senator Bill Cassidy, a physician who expressed skepticism about Kennedy's confirmation and urged that the panel should not convene due to its size and the absence of an acting CDC director to endorse any recommendations. Cassidy remarked on the members' varying scientific backgrounds, pointing out gaps in experience with mRNA technologies and emerging vaccine methodologies.
This recent shake-up calls into question the future of vaccination policies and public health strategies in the United States, as the new panel prepares to make decisions that could significantly impact child health.
The latest members of ACIP include individuals with ties to vaccine skepticism, prompting public health experts and political figures to voice concerns about their qualifications. During the meeting, the panel's chair, Dr. Martin Kulldorff, revealed he was previously terminated from his teaching position at Harvard for refusing to receive a COVID-19 vaccine. He announced plans for new working groups that will investigate childhood vaccination schedules, along with the reassessment of vaccines that gained approval over seven years ago, including the longstanding hepatitis B vaccine administered to newborns.
Concerns surrounding this review are substantial, particularly as critics surmise it undermines the credibility of approved vaccines. Bill Hanage, a professor at Harvard TH Chan School of Public Health, stated that questioning the approval processes of these longstanding vaccinations lacks rational justification. The panel was expected to cast votes regarding new recommendations for RSV, a respiratory virus posing risks to infants, but this agenda item has been postponed.
As part of the meeting, the panel also planned to discuss thimerosal, a mercury-containing preservative that has not been included in most vaccines for many years. This choice has puzzled experts, including Dr. Hanage, who expressed frustration that the new committee lacks the diverse expertise seen in prior panels. Former ACIP member Dr. Paul Offit criticized Kennedy's selection process, indicating that members predominantly share an anti-vaccine stance similar to Kennedy's.
Further criticism comes from Republican Senator Bill Cassidy, a physician who expressed skepticism about Kennedy's confirmation and urged that the panel should not convene due to its size and the absence of an acting CDC director to endorse any recommendations. Cassidy remarked on the members' varying scientific backgrounds, pointing out gaps in experience with mRNA technologies and emerging vaccine methodologies.
This recent shake-up calls into question the future of vaccination policies and public health strategies in the United States, as the new panel prepares to make decisions that could significantly impact child health.