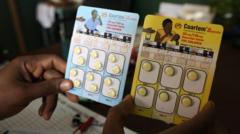
The inaugural malaria treatment specifically designed for infants and young children has received approval and is poised for distribution across African countries in the coming weeks. Historically, there has been no approved medication tailored to treat babies, forcing health professionals to administer medications formulated for older children, which may pose overdose risks due to their differing metabolic rates and physiological development.
Malaria resulted in approximately 597,000 deaths in 2023, with a concerning majority occurring in Africa, particularly among children under five years of age. The absence of infant-specific treatments has created what experts call a "treatment gap," highlighting the urgent need for targeted medical interventions in this vulnerable population.
The newly approved medication, developed by Novartis in collaboration with the Medicines for Malaria Venture (MMV), aims to fill this void. The company plans to introduce the drug, known as Coartem Baby or Riamet Baby in various countries, largely on a non-profit basis, emphasizing its commitment to address health inequality.
Vas Narasimhan, CEO of Novartis, expressed optimism about the drug's potential impact, stating, "This is a significant moment in the fight against malaria. For years, we have remained dedicated to creating breakthroughs that tackle the burden of this disease."
Martin Fitchet, CEO of MMV, also stressed the importance of the approval, noting that malaria is especially fatal for children. Dr. Marvelle Brown, an associate professor at the University of Hertfordshire, lauded the initiative, pointing out the high mortality rate from malaria and the additional complications faced by infants with conditions like sickle cell disease.
This approval and upcoming rollout of Coartem Baby present a crucial opportunity to combat malaria's devastating impact on newborns and young children, reaffirming the global commitment to tackling this preventable disease.
Malaria resulted in approximately 597,000 deaths in 2023, with a concerning majority occurring in Africa, particularly among children under five years of age. The absence of infant-specific treatments has created what experts call a "treatment gap," highlighting the urgent need for targeted medical interventions in this vulnerable population.
The newly approved medication, developed by Novartis in collaboration with the Medicines for Malaria Venture (MMV), aims to fill this void. The company plans to introduce the drug, known as Coartem Baby or Riamet Baby in various countries, largely on a non-profit basis, emphasizing its commitment to address health inequality.
Vas Narasimhan, CEO of Novartis, expressed optimism about the drug's potential impact, stating, "This is a significant moment in the fight against malaria. For years, we have remained dedicated to creating breakthroughs that tackle the burden of this disease."
Martin Fitchet, CEO of MMV, also stressed the importance of the approval, noting that malaria is especially fatal for children. Dr. Marvelle Brown, an associate professor at the University of Hertfordshire, lauded the initiative, pointing out the high mortality rate from malaria and the additional complications faced by infants with conditions like sickle cell disease.
This approval and upcoming rollout of Coartem Baby present a crucial opportunity to combat malaria's devastating impact on newborns and young children, reaffirming the global commitment to tackling this preventable disease.